Understanding Supercooling
Supercooling occurs when a liquid cools below its freezing point without solidifying. For water, this means temperatures below 0°C (32°F) while remaining liquid.
Mechanism Behind Instant Freezing
Water remains supercooled because nucleation sites—like impurities or surfaces—are absent, preventing ice crystal formation. This unstable state relies on the absence of triggers for solidification. Upon disturbance, such as physical agitation, crystals form rapidly, releasing latent heat and causing instantaneous freezing.
Practical Tricks to Achieve Instant Freezing
Key methods to trigger supercooled water:
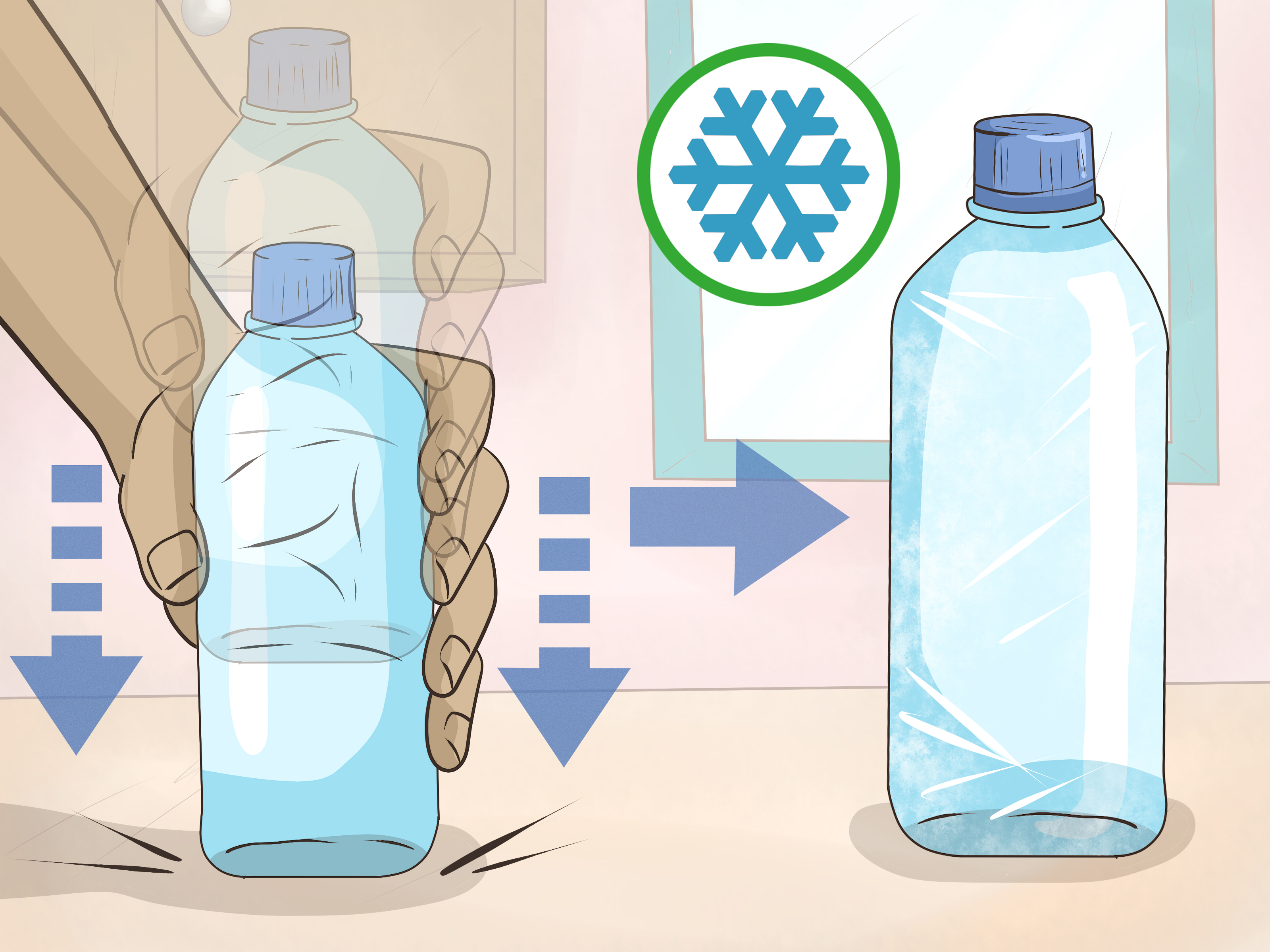
- Physical Shock: Shake or vibrate the container to induce nucleation, instantly converting liquid to ice.
- Introduction of Nucleators: Add a small particle (e.g., dust or ice crystal) to serve as a seed for crystallization.
- Temperature Control: Maintain pure water in a clean, smooth container at -5°C to -15°C before initiating disturbance.
These techniques exploit thermodynamics, requiring precision to avoid spontaneous nucleation for dramatic effects.